Pexa-Vec (JX-594)
Pexa-Vec was the first engineered oncolytic virus with demonstrated intravenous delivery to tumors (Breitbach, Hwang, Kirn et al., Nature 2011), induction of cancer targeting antibodies (Hwang, Kirn et al., Science Translational Medicine 2013) and improved survival in a randomized trial (Heo, Reid, Ruo et al., Nature Medicine 2013).
To-date, over 300 patients with advanced cancers have received Pexa-Vec treatments(www.clinicaltrials.gov). The PHOCUS (www.pexavectrials.com) trial, a global Phase 3 clinical trial to evaluate Pexa-Vec in combination with sorafenib versus sorafenib alone in patients with advanced unresectable hepatocellular carcinoma (HCC) is expected to begin in December 2015. Pexa-Vec was the first engineered oncolytic virus with demonstrated intravenous delivery to tumors (Breitbach, et al., Nature 2011) and induction of cancer targeting antibodies (Hwang,et al., Science Translational Medicine 2013).
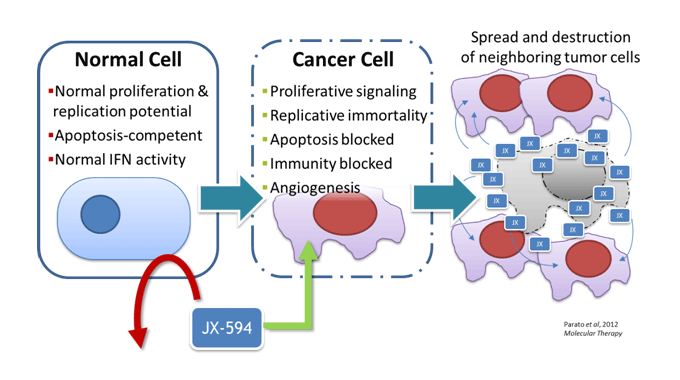
In a small phase 2 dose-randomized study in front line liver cancer (HCC) patients, high dose Pexa-Vec treatment was associated with improved survival in HCC patients. As has been the experience with other immunotherapies, Pexa-Vec treatment did not provide survival benefit in late stage, refractory HCC patients; these results suggest that less advanced patients may be more likely to benefit from an oncolytic immunotherapy such as Pexa-Vec.
Case Study 1. Patient with Hepatocellular Carcinoma
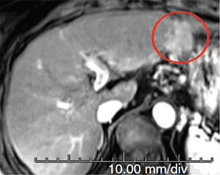 Baseline
Baseline
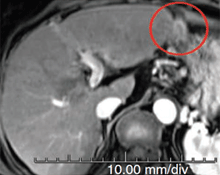 Week 8
Week 8
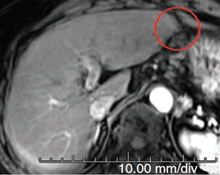 Month 21
Month 21
This patient, presenting with recurrent hepatocellular carcinoma (HCC), was unable to receive TACE or surgery due to risk of gastric perforation. At the time of first visit, the patient had a life expectancy of only 6 months. However, after 3 treatments with Pexa-Vec, the patient exhibited complete tumor response, and the patient remains alive and disease-free over 4 years after treatment initiation. (Nature Medicine 2013)
Case 2. Patient with Hepatocellular Carcinoma
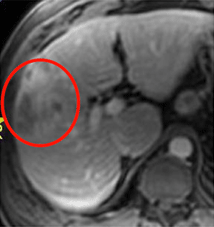 Baseline
Baseline
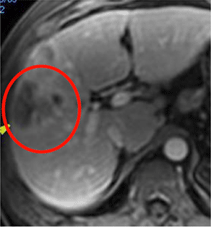 Day 5
Day 5
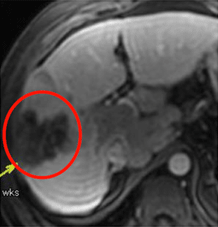 Week 14
Week 14
Baseline: Before treatment, the patient presented with a tumor 10 cm in diameter.
Day 5 & Week 14: Antitumor response was evaluated by dynamic MRI. Example of the effects of Pexa-Vec on contrast enhancement and perfusion in an injected tumor over time. (Nature Medicine 2013)
